Health and Healthcare
How Inovio Pharma Is Joining the Fight Against the Zika Virus
Published:
Last Updated:

Inovio Pharmaceuticals Inc. (NASDAQ: INO), in conjunction with GeneOne Life Science, is pioneering a new treatment for the Zika virus. In fact, early Monday morning the companies announced that they have received approval to initiate a Phase 1 human trial to evaluate Inovio’s Zika DNA vaccine (GLS-5700) to prevent infection from this concerning virus.
In preclinical testing, this synthetic vaccine induced what the companies are calling a robust antibody and T-cell responses in small and large animal models. They further believe that this demonstrates the product’s potential to prevent infection from this harmful pathogen in humans.
Inovio and GeneOne are developing the Zika vaccine, GLS-5700, with academic collaborators from the United States and Canada with whom they have previously collaborated to advance Inovio’s Ebola and MERS vaccines into clinical development.
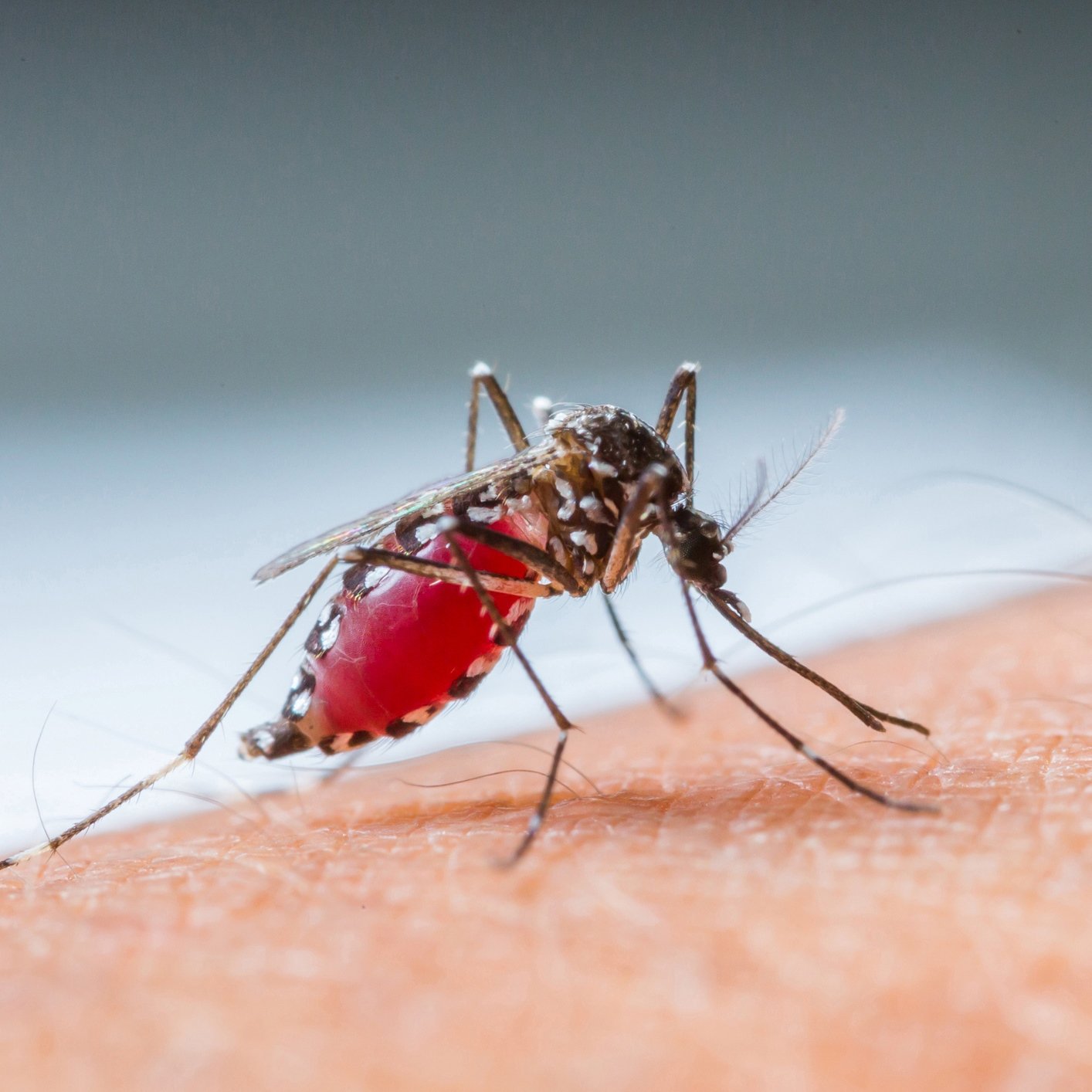
The Zika virus is a flavivirus, a family of viruses that are introduced to people through mosquito bites. It also can be sexually transmitted. The most common symptoms of Zika virus are fever, rash, joint pain and conjunctivitis.
More seriously, Zika has been linked to a severe birth defect called microcephaly, which arises from infection during pregnancy. Microcephaly is a rare condition marked by an abnormally small head and incomplete brain development. Zika is also associated with Guillain-Barré syndrome, which causes muscle weakness of the limbs and in severe cases may cause almost total paralysis, including the inability to breath.
Dr. J. Joseph Kim, Inovio’s president and CEO, commented:
We are proud to have attained the approval to initiate the first Zika vaccine study in human volunteers. As of May 2016, 58 countries and territories reported continuing mosquito-borne transmission of the Zika virus; the incidences of viral infection and medical conditions caused by the virus are expanding, not contracting. We plan to dose our first subjects in the next weeks and expect to report phase I interim results later this year.
So far in 2016, Inovio has outperformed the broad markets, with the stock up 55.8%, as of Friday’s close.
Shares of Inovio closed Friday down 2.5% at $10.47, with a consensus analyst price target of $15.50 and a 52-week trading range of $4.50 to $11.69. Following the announcement, the stock was up 3.7% at $10.86 in early trading indications Monday.
Want retirement to come a few years earlier than you’d planned? Or are you ready to retire now, but want an extra set of eyes on your finances?
Now you can speak with up to 3 financial experts in your area for FREE. By simply clicking here you can begin to match with financial professionals who can help you build your plan to retire early. And the best part? The first conversation with them is free.
Click here to match with up to 3 financial pros who would be excited to help you make financial decisions.
Thank you for reading! Have some feedback for us?
Contact the 24/7 Wall St. editorial team.